Refrigeration, a cornerstone of modern society, has revolutionized food preservation and transformed our daily lives. From humble beginnings to sophisticated technologies, refrigeration has evolved to become an indispensable part of our kitchens, industries, and scientific endeavors. But have you ever wondered how this remarkable process works? Delve into the science behind refrigeration as we unravel the secrets of keeping things cool.
The Science of Keeping Cool: Understanding the Refrigeration Cycle
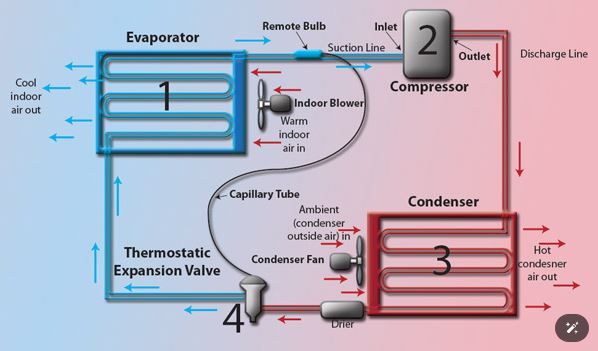
At the heart of refrigeration lies a thermodynamic cycle that involves the transfer of heat. This cycle, known as the vapor-compression refrigeration cycle, consists of four main stages:

Evaporation:
The evaporation stage is the cornerstone of refrigeration’s cooling magic. Here’s where the chosen refrigerant, a marvel of science with a low boiling point, enters the scene. Imagine this low-pressure refrigerant as a tiny heat sponge. As it flows through the evaporator coil, it readily absorbs heat from the surrounding environment within the refrigerator. This process is similar to how sweat evaporates from your skin, leaving you feeling cooler. The key difference here is that the refrigerant doesn’t disappear – it undergoes a phase change, transforming from a cool liquid into a low-pressure gas, a vital step in the refrigeration cycle.
This phase change from liquid to gas in the evaporator coil is crucial for refrigeration’s effectiveness. Remember, the refrigerant absorbs heat during this transformation. The heat absorbed isn’t “used up” by the refrigerant; it’s essentially carried away by the refrigerant as it transitions into a gas. This allows the evaporator coil to continuously extract heat from the refrigerator’s interior, maintaining a consistently cool environment for your food and beverages. The now-gaseous refrigerant, carrying its load of absorbed heat, is ready for the next stage of the journey: compression.
Compression:
The compressor, aptly named the “heart” of a refrigeration system, takes center stage in this next phase. Imagine this powerful component as a high-tech pump specifically designed for refrigerants. The low-pressure, heat-laden vapor from the evaporator is drawn into the compressor. Here, the magic happens – the compressor squeezes the refrigerant vapor, significantly increasing its pressure. This forceful compression isn’t just about force; it has a surprising side effect: a dramatic rise in temperature. According to the laws of physics, compressing a gas traps its internal energy, leading to a significant increase in temperature. While this might seem counterintuitive in our quest for coolness, it’s a crucial step. The now-hot, high-pressure refrigerant is perfectly primed for the next stage – releasing its accumulated heat to the environment.
The compression process within the compressor transforms the refrigerant vapor in two key ways. First, the pressure skyrockets, creating a high-energy state. Second, and perhaps more importantly for our cooling goals, the refrigerant temperature also rises significantly. This temperature increase might seem counterproductive, but it’s a clever trick. Concentrating the heat within the refrigerant vapor prepares the compressor for the next vital step: releasing this accumulated heat to the surrounding environment. The now-superheated refrigerant vapor is ready to travel to the condenser coil, where it will shed its heat burden, paving the way for continued cooling within the refrigerator.
Condensation:
The hot, high-pressure refrigerant vapor that emerges from the compressor embarks on a crucial journey. Its destination: the condenser coil, typically located on the back or bottom of your refrigerator (and often the part that feels warm to the touch). Imagine the condenser coil as a giant heat exchanger. The hot refrigerant vapor, carrying the heat it absorbed earlier in the evaporator, travels through this intricate network of tubes. Here, a critical exchange takes place.
The condenser coil, strategically positioned to interact with the surrounding air (either through natural convection or aided by a fan), acts as a heat sink. The hot refrigerant vapor releases the heat it has been carrying to the cooler surrounding air. This heat transfer process causes the refrigerant vapor to undergo another phase change – it condenses back into a liquid state. The condenser coil, through its efficient heat dissipation, effectively removes the unwanted heat from the refrigeration system, paving the way for the refrigerant to return to its cooling duties.
The transformation that occurs within the condenser coil is nothing short of remarkable. The high-pressure, high-temperature refrigerant vapor that arrived from the compressor sheds its heat burden to the surrounding air. This heat transfer has a profound effect. As the refrigerant vapor releases its thermal energy, it cools down significantly and condenses back into a liquid state. This condensed liquid refrigerant is now cooler and at a much higher pressure compared to its state at the beginning of the cycle. The stage is now set for the final act: a pressure drop that prepares the cool liquid refrigerant to re-enter the evaporator and absorb heat once again, perpetuating the ingenious refrigeration cycle.
Expansion:
The expansion valve, a seemingly simple component within the refrigeration system, plays a vital role in maximizing cooling efficiency. Imagine this valve as a strategic checkpoint for the refrigerant. The high-pressure liquid refrigerant, fresh from its heat-shedding experience in the condenser coil, arrives at the expansion valve. Inside the valve, a controlled pressure drop takes place. This might seem like a minor adjustment, but it has a significant impact on the refrigerant’s temperature. According to the principle known as the Joule-Thomson effect, when a gas (or in this case, a high-pressure liquid with some gaseous properties) undergoes a rapid pressure drop, its temperature decreases. This scientific principle is harnessed by the expansion valve to give the refrigerant a much-needed temperature plunge.
The pressure drop orchestrated by the expansion valve has a transformative effect on the refrigerant. As the pressure falls dramatically, the refrigerant experiences a significant drop in temperature. This temperature reduction is essential for the refrigerant to return to its heat-absorbing duties within the evaporator coil. Imagine the refrigerant like a sponge; high pressure squeezes out its ability to absorb heat, while a pressure drop allows it to “re-expand” and regain its cooling capacity. The now-chilled, low-pressure liquid refrigerant is perfectly suited to re-enter the evaporator coil, ready to absorb heat from the refrigerator’s interior and kickstart the cooling cycle all over again. This continuous cycle of evaporation, compression, condensation, and expansion is the magic behind refrigeration, ensuring our food and beverages stay perfectly chilled.
Repeat:
The beauty of refrigeration lies in its cyclical nature. The cooled, low-pressure liquid refrigerant exiting the expansion valve embarks on a return journey, ready to repeat its heat-absorbing mission. Imagine the evaporator coil as a waiting station for the renewed refrigerant. Here, the chilled liquid refrigerant re-enters the evaporator coil, eager to absorb heat once again. This closed-loop system ensures a continuous flow of heat transfer. As the refrigerant circulates through the evaporator coil, it readily absorbs heat from the surrounding environment within the refrigerator.
This heat absorption process lowers the temperature inside the refrigerator, keeping your food and drinks perfectly chilled. The now-warmed, low-pressure refrigerant vapor has completed its loop and is ready to travel back to the compressor, where it will be squeezed and heated, initiating the heat rejection process all over again. This remarkable cycle of heat absorption and rejection is the heart of refrigeration, a testament to human ingenuity in creating a controlled cool environment.
The seemingly simple act of keeping your food cold relies on a well-coordinated dance between the various components of a refrigerator. The cooled liquid refrigerant re-entering the evaporator coil acts as the conductor, initiating the next round of heat absorption. As the refrigerant absorbs heat, it transforms back into a vapor, carrying the thermal energy away from the refrigerator’s interior. This heat-laden vapor is then drawn into the compressor, where it’s pressurized and heated. The hot, high-pressure vapor travels to the condenser coil, where it sheds its heat burden to the surrounding air. The cooled, high-pressure liquid refrigerant then journeys through the expansion valve, experiencing a pressure drop that triggers a significant temperature decrease. This continuous cycle, where each component plays a crucial role, ensures efficient and reliable cooling, keeping your food fresh and beverages perfectly chilled.
The Role of Key Components: A Symphony of Refrigeration
While the refrigeration cycle provides the theoretical framework, several key components work together to make it a reality:
- Compressor: The compressor, the “heart” of the system, is responsible for compressing the refrigerant vapor, raising its temperature and pressure.
- Evaporator Coil: The evaporator coil, located inside the refrigerator compartment, absorbs heat from the surrounding air, causing the refrigerant to evaporate.
- Condenser Coil: The condenser coil, usually placed on the exterior of the refrigerator, releases heat from the compressed refrigerant vapor to the surrounding air.
- Expansion Valve: The expansion valve regulates the flow of refrigerant, allowing it to expand and cool down before re-entering the evaporator coil.
Applications of Refrigeration: Beyond the Kitchen
Refrigeration’s reach extends far beyond our kitchens, playing a crucial role in various industries and scientific fields:
- Food Preservation: Refrigeration is essential for preserving food by slowing down the growth of microorganisms that cause spoilage.
- Air Conditioning: Refrigeration principles are applied in air conditioning systems to cool and dehumidify indoor air.
- Medical and Pharmaceutical Applications: Refrigeration is vital for storing and transporting temperature-sensitive medical supplies and pharmaceuticals.
- Industrial Applications: Refrigeration is used in various industrial processes, such as chemical manufacturing and metalworking.
- Scientific Research: Refrigeration plays a critical role in scientific research, enabling experiments and studies that require precise temperature control.
The Future of Refrigeration: Embracing Sustainability and Efficiency
As we strive for a more sustainable future, the refrigeration industry is constantly innovating to reduce its environmental impact and improve energy efficiency:
- Natural Refrigerants: Replacing traditional refrigerants with eco-friendly alternatives like hydrofluorocarbons (HFCs) and natural refrigerants like ammonia and carbon dioxide.
- Energy-Efficient Technologies: Developing more efficient compressors, insulation materials, and control systems to minimize energy consumption.
- Smart Refrigeration: Incorporating smart technologies to optimize refrigeration performance, reduce energy waste, and monitor food storage conditions.
Conclusion: Refrigeration – A Technology that Transforms
Refrigeration stands as a testament to human ingenuity, transforming our ability to preserve food, control temperatures, and advance scientific endeavors. From humble beginnings to cutting-edge technologies, refrigeration continues to evolve, shaping our world profoundly. As we embrace sustainable practices and strive for energy efficiency, the future of refrigeration promises even greater advancements, ensuring a cooler, more sustainable future for all.
FAQ:
- What are the different types of refrigerators?
There are common refrigerator types on HVAC systems include household refrigerators, commercial refrigerators, medical refrigerators, and industrial refrigerators. Each type is designed for specific applications and temperature requirements.
- How does a refrigerator maintain a constant temperature?
A thermostat inside the refrigerator controls the compressor, turning it on and off to maintain the desired temperature.
- What are some tips for using my refrigerator efficiently?
- Avoid overloading the refrigerator, allowing for air circulation.
- Keep the door closed as much as possible.
- Regularly clean the condenser coils to ensure proper heat transfer.